The Rapid Emergence Of COVID-19 Vaccines
“Moderna Vs. Pfizer”
Image by torstensimon from Pixabay
January 26, 2021
As we enter the year 2021, there is still hope for an end to the infamous COVID-19 pandemic. As of Jan. 23, 2021, there were 25,383,300 total cases of coronavirus worldwide, a total of 424,120 citizens died (1.67%) and a total of 15,222,295 people have recovered. Throughout history, many viruses spread across the world and shocked the nations including Ebola, Influenza, HIV, SARS-CoV-1 and now SARS-CoV-2. Although many scientists tirelessly work to provide definitive vaccines, there are absolutely none guaranteed to protect us. These viruses have occurred – and will occur again – in the future. Unfortunately, we have lived to witness such a history and experience it like previous generations, whom we only read about in our history books. This pandemic is no different, and it requires immediate protection and security. Some companies took these harsh matters into their own hands to provide immediate help to the people by asking the U.S Food and Drug Administration to grant them the right to present their vaccines to the world.
On Dec. 11, 2020, Pfizer-BioNTech COVID-19 vaccine was the first company that was granted Emergency Use Authorization (EUA). Only seven days later, Moderna was also granted this authorization. Both are part of the US government’s Operation Warp Speed (OWS) program, a partnership among components of the Department of Health and Human Services (HHS), including the Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH), and the Biomedical Advanced Research and Development Authority (BARDA), and the Department of Defense (DoD). OWS engages with private firms and other federal agencies, including the Department of Veterans Affairs. It will coordinate existing HHS-wide efforts, including the NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership, NIH’s Rapid Acceleration of Diagnostics (RADx) initiative, and work by Biomedical Advanced Research and Development Authority (BARDA).
Distribution of Moderna and Pfizer-BioNTech, including prioritization and available volume, is being managed by the federal government in coordination with state, territorial, tribal, and local health departments.
Both vaccines are mRNA vaccines. This means that part of the coronavirus’ genetic code is injected into the body, triggering the body to begin making viral proteins but not the whole virus, which is enough to train the immune system to attack. These types of vaccines are new and have never been presented or used in the general population before. “CoronaVac is a more traditional method of vaccine that is successfully used in many well-known vaccines like rabies,” said Associate Professor Luo Dahai of the Nanyang Technological University. Although both vaccines have been granted with Emergency Use Authorization (EUA) by the FDA in hopes of preventing the spread of COVID-19, both vaccines are still officially unapproved by the FDA.
Common Safety Information
The Pfizer-BioNTech COVID-19 and Moderna vaccines have not been approved or licensed by the FDA, but have been authorized for emergency use under an Emergency Use Authorization to prevent Coronavirus Disease 2019 (COVID-19), eligible for use in individuals 16 years of age and older for Pfizer and 18 years of age and older for Moderna. The emergency use of these products is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of the medical products under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act (FD&C), unless the declaration is terminated or authorization revoked sooner.
Pfizer-BioNTech
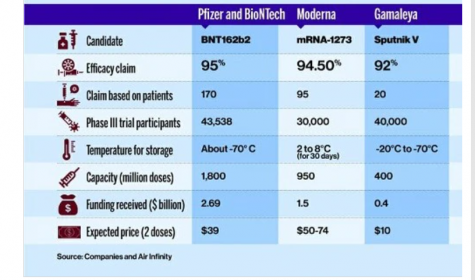
The first coronavirus vaccine authorized for emergency use – the drug produced by German biotech firm BioNTech and US pharmaceutical giant Pfizer – has also been approved for use in the UK and Saudi Arabia, as well as at least 19 other countries. The drug is being produced at BioNtech and Pfizer facilities in Germany and Belgium as well as in other countries. The Pfizer-BioNTech COVID-19 vaccination is administered intramuscularly as a series of 2 doses (0.3 mL each) 3 weeks apart and must be stored at -94 degrees Fahrenheit. Although Pfizer’s clinical trials are still ongoing, their vaccine efficacy analysis were finalized at 95% efficacy, involving 170 patients. Pfizer plans to produce as many as 1.3 billion doses for global use in 2021. Their fact sheet is available to the public on their website.
Potential Risks and Issues
- Currently, only 2 months of statistical data available from clinical trials
- No long term data available
- Final efficacy analysis involved only 170 patients
- There is no data available on the interchangeability of the Pfizer-BioNTech COVID-19 vaccine with other COVID-19 vaccines to complete the vaccination series.
- Individuals with a history of severe allergic reaction (e.g., anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 vaccine must be careful
- The Pfizer-BioNTech COVID-19 vaccine may not protect all vaccine recipients
- During the 2 months of clinical studies, adverse reactions in participants 16 years of age and older included: pain at the injection site (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%), injection site swelling (10.5%), injection site redness (9.5%), nausea (1.1%), malaise (0.5%), and lymphadenopathy (0.3%)
- Available data on Pfizer-BioNTech COVID-19 vaccine administered to pregnant women are insufficient for determining whether there are vaccine-associated risks during pregnancy
- Data are not available to assess the effects of Pfizer-BioNTech COVID-19 vaccine on the breastfed infant or on milk production/excretion
- The duration of protection against COVID-19 is currently unknown
- All possible side effects are still unknown and serious and unexpected side effects may occur
- This vaccine still being studied in clinical trials
Moderna
Biotech company Moderna was founded in 2010 and was formerly named ModeRNA Therapeutic. Currently, the firm is based in Cambridge, Massachusetts and owned by shareholders. The firm is planning to produce about 1 billion COVID-19 vaccines. Although the vaccine may not protect everyone, the European Union has already ordered 80 million doses of the vaccine with an option of buying an additional 80 million, while the US has secured 200 million doses with the option of purchasing another 300 million. The UK, Japan, Switzerland, and South Korea have also reserved doses. Canada, which has also approved the drug, has reserved 40 million doses. The vaccine is administered into a muscle as a 2-dose series (0.5 mL each), and 1 month apart. Vaccine efficacy of 94.5% has been demonstrated at the first interim analysis with a total of 95 cases. Their fact sheet is also available to the public on their website.
Potential Risks and Issues
- The data do not provide sufficient information about longer term protection beyond 2 months
- Vaccine effectiveness against asymptomatic infection is limited
- Vaccine has not been studied and is not authorized for use in individuals younger than 18 years of age
- The adverse reactions in participants were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%)
- There were three reports of facial paralysis (Bell’s palsy) after the second dose
- The duration of protection against COVID-19 is unknown
- The evolution of the pandemic characteristics may potentially limit the generalizability of the efficacy conclusions over time.
- Safety in certain subpopulations is limited
- Data on vaccine administered to pregnant women are ongoing for determining whether there are vaccine-associated risks during pregnancy
- All possible side effects are still unknown and serious and unexpected side effects may occur
- This vaccine still being studied in clinical trials
Both vaccines are still being studied and therefore, neither is confident in absolute immunity. After the second dose of either vaccine, individuals must still stand 6ft apart in public settings and must continue to wear their masks. Unfortunately, vaccines alone cannot guarantee the safety from COVID-19. Health care workers and elderly population are first in line to receive the vaccines. If both vaccines are available, individuals can choose whichever one they prefer. Based on clinical studies, Moderna participants reported higher on injection site pain than Pfizer participants, but lower allergic reactions to the vaccine than Pfizer participants.
The PREP Act
In Feb. 2020, the U.S. Health and Human Services Secretary Alex Azar invoked the PREP Act to shield drugmakers from liability for injuries people suffer from treatments and vaccines including COVID-19 vaccines. A Public Readiness and Emergency Preparedness (PREP) Act declaration is specifically for the purpose of providing immunity from liability, and is different from, and not dependent on, other emergency declarations. Pfizer and Moderna specifically are protected under this declaration from lawsuits and cannot be sued for any damages except for death or serious physical injury, and only if caused purposefully by protected entities under the declaration. Individuals are not guaranteed to be eligible for compensation but may try through the Countermeasures Injury Compensation Program. In the past 10 years, the Countermeasures Injury Compensation Program has rejected 90% of its vaccine related injury claims.
Both Pfizer and Moderna are going in the right direction. However, under these exceptional circumstances, it leaves a lot of room for uncertainty and growth. Two main factors are at stake – one, being time and two, effectiveness. In order for a vaccine to be considered effective, it needs time to establish itself through trials, which are currently ongoing and may continue into the year 2023. Until then, the effectiveness of these vaccines may either increase or decrease over time. Only time will tell.